
| Home | Table of contents | Keys | Species list | Glossary | Image data | PDF | Cite this article | Feedback | Updates |
Identification Atlas of the Vespidae (Hymenoptera, Aculeata) of the northeastern Nearctic region
CJAI 05, February 19, 2008
doi: 10.3752/cjai.2008.05
Matthias Buck, Stephen A. Marshall, and David K.B. Cheung
Department of Environmental Biology, University of Guelph, Guelph, Ontario, Canada N1G 2W1
4. Morphology
Terminology | Identification of sexes
Cephalic foveae | Flagellum | Mandible | Costal scale | Femoral pubescence | Fore tarsus | Acarinarium | Sternum 2
Terminology. All morphological terms are explained in the appended glossary. Most structures are illustrated and labelled in the Figures below.
Head 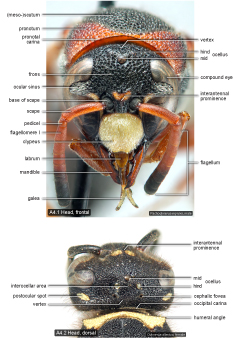 |
Body, lateral  |
Body, dorsal 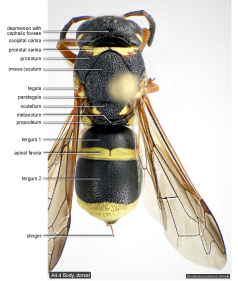 |
Wing cells 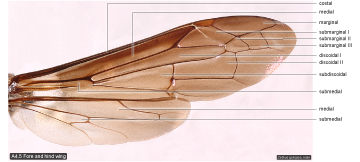 |
Wing veins 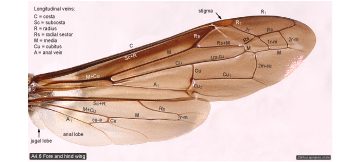 |
Identification of sexes. Many diagnostic characters in Vespidae show sexual dimorphism. When using the keys it is therefore necessary to determine the sex of the specimen to be identified. Males can be distinguished from females as follows.
Male: Flagellum (antenna excluding two basal segments) almost always consisting of 11 flagellomeres (last flagellomere absent in Pachodynerus erynnis; apical flagellomeres more or less fused and difficult to distinguish in Masarinae; Figs B1.5, B1.16); flagellum often hooked or coiled apically (e.g., Figs B2.24, B2.8). Metasoma with 7 externally visible segments (Fig. 4.3).
Female: Flagellum with 10 flagellomeres, never hooked or coiled apically. Metasoma with 6 externally visible segments.
In the field, male Eumeninae can usually be distinguished from females by their yellow clypeus; females usually have a predominantly black clypeus (in a few species males show substantial black markings [e.g., Pseudodynerus quadrisectus], whereas in others females may have an entirely yellow clypeus [e.g., Ancistrocerus lutonidus). In most male Polistes the clypeus is entirely yellow whereas females have at least some black or ferruginous markings (not applicable to some species from other parts of North America). Sexing Vespinae in the field is slightly more difficult, though the experienced observer will easily recognize males by their longer antennae and their more elongate metasoma.
New diagnostic characters and morphological observations
Cephalic foveae. Cephalic foveae are unique morphological structures that evolved within the Eumeninae (Carpenter and Cumming 1985). These usually paired pits located behind the ocelli on the vertex (e.g., Fig. 4.2) represent openings of dermal glands of unknown function. So far they have only been recorded from female wasps (Carpenter and Cumming 1985, Cumming and Leggett 1985). During this study cephalic foveae were discovered for the first time in male Eumeninae, in several species of the closely related genera Parancistrocerus and Stenodynerus (we have also seen cephalic foveae in males of two unidentified species of Montezumia from Bolivia). The foveae of male wasps are always smaller and less conspicuous than in females of the same species, and are usually not located in a conspicuous depression as in females. In some cases the functionality of male foveae is evidenced by small amounts of dried gland secretions in and around the foveae, as found in female wasps. In some species the development of male foveae is variable, and they may not be readily detectable in all specimens. A definitive determination of presence/absence is sometimes difficult to make by external examination because the usually very small foveae are hard to detect among the rough surface sculpture of the vertex. In these cases only dissection could yield unambiguous results but this was not attempted here. Cumming and Leggett (1985) report cephalic foveae in male-like intersex specimens without mentioning a taxon. The males examined here all appear to be normal males. Cephalic foveae were found in males of the following species: Parancistrocerus salcularis (Figs 4.8, 4.10), P. bicornis, P. fulvipes, P. toltecus (de Saussure), P. vagus, P. leionotus (distinct only in some specimens), Stenodynerus fundatiformis (foveae coalescent in female, separated in male), S. propinquus (de Saussure) (as in previous species), S. toas (Cresson), S. ammonia paraensis (often indistinct), and S. histrionalis. More species could probably be added to this list through careful examination. The presence of cephalic foveae in both sexes has interesting implications for the possible function of these structures. Their presence in both males and females makes it less likely that they secrete pheromones involved with mating behaviour.
Cephalic foveae have been used in eumenine taxonomy before but not for the species treated in this Atlas. We found them to be crucial for diagnosing species in the difficult Ancistrocerus catskill complex (Figs B3.12–13).

Flagellum. The vestiture of the flagellum has to our knowledge not been used in eumenine taxonomy. We found the presence/absence of small, spine-like hairs on the male flagellum a useful diagnostic character for species in the Euodynerus castigatus-group (Fig. B5.27). The length of hairs on the basal flagellomeres of Polistes fuscatus-group females helped to recognise one undescribed species (Figs B10.18–19).
The flagellum of female Eumeninae offers very few characters of taxonomic value. The relative length of the basal flagellomere was found here to be useful as an additional character to separate females of Symmorphus.
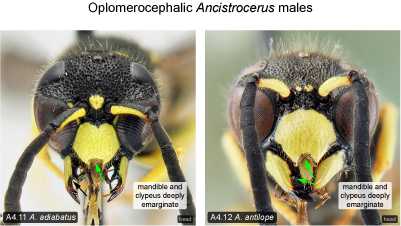
Mandible. Some male Eumeninae show conspicuous, sexually dimorphic modifications of the mandible such as a deep notch between mandibular teeth 2 and 3, combined with a deep apical emargination of the clypeus (e.g., Odynerus dilectus, Pseudepipona; corresponding females have the mandibular teeth evenly spaced and the clypeus at most slightly emarginate). As a very rare aberration this condition is also found in some species of Ancistrocerus. We have examined five males of A. adiabatus (Fig. 4.11) from Ontario, Quebec and North Carolina (CNCI, DEBU, LEMQ) and one male of A. antilope (Fig. 4.12) from Virginia (DEBU) with both mandibles and clypeus modified. Blüthgen (1958) coined the term ‘oplomerocephaly’ (“Oplomerocephalie”) for this condition, which he suspected to be caused by parasitism by Mermithidae (Nematoda). Blüthgen (1944, 1958, 1961, 1966) mentions three Palaearctic Ancistrocerus species in which oplomerocephalic specimens have been found: A. nigricornis (Curtis), A. parietinus (L.), and A. oviventris (Wesmael). In males of the Mexican A. arista (de Saussure) the mandible is always emarginate (Carpenter and Cumming 1985). To our knowledge oplomerocephalic males have not been recorded previously from any Nearctic species with otherwise normally developed mandibles. It is likely that occasional oplomerocephalic specimens will be found in other Nearctic Ancistrocerus species than the ones mentioned here.
The mandible shows less variation within Eumeninae females and has therefore not been used to characterize species treated in this Atlas. We found that the length of mandibular teeth can be diagnostic in the genera Euodynerus and Stenodynerus. The apical tooth is more elongate in two ground-nesting species of Euodynerus (Fig. B5.28) and one ground-nesting Stenodynerus (Fig. B7.37). The two Euodynerus species with elongate teeth (E. annulatus, E. crypticus) often nest in hard-packed soil (Isely 1914), while another ground-nesting species with shorter teeth (E. auranus) nests in sandy soils. Perhaps the elongation of the apical tooth evolved in response to the greater wear by excavating in hard-packed soils.
Costal scale of fore wing. This character is employed here for the first time in eumenine taxonomy. Richards (1978) used the same structure, which he referred to as the “keel beneath base of costa”, to key out one species of Agelaia (Polistinae: Epiponini). The costal scale is a small scale- or ridge-like ventral elevation at the very base of the costa (Figs B3.10–11). Its shape is of crucial taxonomic importance in the difficult Ancistrocerus catskill-complex. Distinct differences between species can also be observed in Euodynerus but a significant number of other, more obvious characters renders the costal scale of little importance for the taxonomy of this genus.
Femoral pubescence. The pubescence below the femora has occasionally been used in eumenine taxonomy (e.g., MacLachlan 1980: Eumenes). Pubescence below the fore and sometimes the hind femur is often sexually dimorphic, and has proven very useful for separating males in the genera Euodynerus and Stenodynerus (e.g., Figs B5.12–13, B7.12–13). Differences of pubescence below the hind femur have helped resolve previous problems in identifying certain species of Symmorphus (Figs B8.6–7; see discussion under S. canadensis).
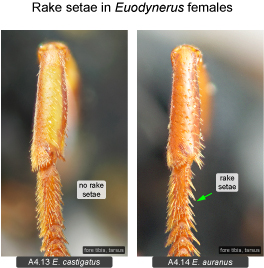
Fore tarsus. A character that has apparently never been reported before is the presence of rake setae in ground-nesting Eumeninae. Rake setae are commonly found in other ground-nesting aculeate wasps such as Pompilidae, Sphecidae, Crabronidae and Mutillidae (e.g., Evans 1950, Bohart and Menke 1976). Within the area under study only two species of Euodynerus have well-developed rake setae on the fore basitarsus (E. auranus: Fig. 4.14, Euodynerus sp. G). Euodynerus auranus nests in dune areas (in Ontario mostly along Lake Huron). The nesting habits of Euodynerus sp. G are unrecorded but probably similar to the closely related E. auranus (with which it was previously confused). The rake setae apparently represent an adaptation for digging burrows in friable sand (Bohart and Menke 1976). Ground-nesting Eumeninae that nest mostly in hard-packed soils (e.g., Euodynerus annulatus, E. crypticus) lack rake setae, and use a completely different digging method. Similar to species that use mud for cell construction, they moisten the ground with water from their crop prior to digging. This requires frequent trips to nearby water sources throughout the process (e.g., Isely 1914). Earth is mostly removed by action of the mandibles, shaped into small pellets and discarded during short flights in close proximity to the nest. The digging method of E. auranus is similar to other ground-nesting aculeate wasps in that water is not used for moistening the ground (unnecessary for digging into friable sand), and that soil is removed through the action of the legs (see Biology section under that species). Interestingly, another eumenine genus that nests in sandy soils, the mostly western Pterocheilus, evolved a different system of soil removal. Here the labial palpi are enlarged and fringed with long bristles forming a basket (‘psammophore’) which is used to carry sand (Carpenter and Cumming 1985: Fig. 16).
Acarinarium. The ‘mite compartment’ between terga 1 and 2 of Parancistrocerus species, and the homologous non-mite bearing structure in the closely related genus Stenodynerus shows important taxonomic features (first used for diagnostic purposes by Bohart 1952). Parancistrocerus vagus differs from congeners in having a small median depression of tergum 2 (Fig. B7.29) that appears to serve as an entrance/exit to the acarinarium. Interestingly, this depression shows a previously unrecorded sexual dimorphism. In the male the depression is wide and relatively shallow whereas in the female it is narrower and deeper. Furthermore, the hind margin of the overhanging recurved basal lamella of tergum 2 forms a wider gap with the remainder of the tergum in the male than in the female, so that the acarinarium is more open to the outside.
Sternum 2. Sternum 2 shows several important diagnostic characters (e.g., ridging of the transverse basal groove, Figs B5.38–39; presence/absence of median furrow). Presence vs. partial absence of pubescence on this sternum is also useful to distinguish certain species of Ancistrocerus (A. lutonidus vs. A. catskill; A. adiabatus vs. A. albolacteus), though in some species this character is variable.
Continue with: Biology
| Home | Table of contents | Keys | Species list | Glossary | Image data | PDF | Cite this article | Feedback | Updates |
